You have no items in your shopping cart.
Lithium-ion cells have the features of highest energy density, low self discharge rate, long life, light weight,and environment friendly, and are widely used for many consumer products and energy storage, from electric vehicles to energy power storage station. However, people are also terrified with the spontaneous combustion and explosion of batteries, which is still a topic that cannot be avoided in the industry. Thermal runaway is the main object of research on improving the safety of lithium-ion batteries.
Thermal runaway refers to the chain reaction phenomenon caused by various causes while the large amount of heat and harmful gases emitted will cause the battery to catch fire and explode. Thermal ranaway is usually caused by three kinds of abuse which are mechanical abuse, electrical abuse, and thermal abuse.
Mechanical abuse- the battery is subjected to external forces such as collision, extrusion, and acupuncture, which ruptures the internal diaphragm(SEI film - solid electrolyte interface) of the lithium battery, exposes the positive electrode to be connected to the negative electrode, and causes an internal short circuit.
Electrical abuse- the battery is subject to internal and external short circuits, overcharge, discharge. Once the lithium battery has internal short circuit, a large amount of heat will be released, chemical reactions under higher temperature conditions will be triggered, these reactions further release heat, which is equivalent to constantly heating the battery.
Thermal abuse- refers to the external heating of the battery, and once the heat inside the battery accumulates to a certain extent, thermal runaway erupts.
Practically, these three occasions are linked and happen in chain.

To learn better on the thermal runaway, let’s see the lithium batter internal structure.
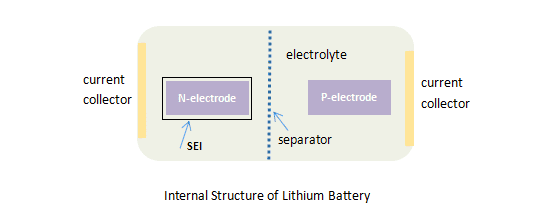
A lithium battery is composed of a positive electrode, a negative electrode and electrolyte. Inside the lithium battery, there is a separation between the positive electrode and the negative electrode, and there is no direct reaction from the two electrodes, mainly the chemical reaction of the electrodes themselves and between their respective electrolytes.
Reaction between positive electrode and electrolyte - The positive electrode material is developed on the basis of transition metal oxides. The thermal decomposition of the positive electrode will release a large amount of oxygen, which is an essential factor for thermal runaway. Carbon dioxide CO2 will be produced in large quantities due to the presence of oxygen, and there will be smoke C2H5F at the same time.
Reaction between negative electrode and electrolyte - The surface of the negative electrode is formed with a SEI film (solid electrolyte interface), which is produced during the first charge and discharge of the battery and is crucial to prevent the direct reaction between the negative electrode and the electrolyte. Once the SEI is decomposed, the negative electrode will be directly exposed to the electrolyte and react with the electrolyte.
Reaction in the electrolyte- In addition to the reaction between the electrodes and the electrolyte, the electrolyte itself will also decompose.
The different chemical reactions inside the lithium battery produce various solid products and gases, and release a lot of heat. Once thermal runaway occurs, its termination can only be the complete combustion of reactants. The battery contains high-energy matters in a closed case, and fire-fighting cannot stop the ongoing thermal runaway for the time being.
In the early stage of thermal runaway of lithium-ion batteries, due to the slow changes in characteristic identification parameters such as battery temperature, discharge voltage, and discharge current, battery failures cannot be detected early through modern BMS(battery management system). And at this time, the electrochemical reaction inside the battery will produce a large amount of gaseous substances.
Therefore, it is theoretically feasible to use gas detection sensors to realize early warning of thermal runaway of lithium-ion batteries. From the internal chemical reactions of lithium batteries, it is found that various gases to emit during the heating process: O2, CO2, C2H4, C4H10, POF3, C2H5F, PF5, H2, etc.
However, lithium batteries also produce some gases during normal operation, such as CO, C2H4 and C4H10, which are different from the gases produced when the temperature rises. According to the significant differences, such as gas content, gas change rate, and gas type comprehensive monitoring can achieve more accurate early warning of lithium battery thermal runaway. In practical applications, it is necessary to select targeted sensors and set corresponding thresholds for judgment.
At present, some companies have made related products in the direction of gas detection combined with fire protection. Winsen sensors for thermal runaway detection are widely used for lithium battery, electric vehicles, energy storage battery pack and station, timely detect and monitor the change of carbon dioxide CO2, carbon monoxide CO, hydrogen H2, smoke, temperature, etc.
Winsen Sensors for battery thermal runaway
CO GAS SENSORS
H2 GAS SENSORS
SMOKE GAS SENSORS

MQ-2 Semiconductor Sensor for Smoke & Flammable Gas
- Flammable gas,Smoke
- 300-10000ppm(Flammable gas)
- READ MORE
 English
English















